The Principal Quantum Number Indicates What Property of an Electron
The electron affinity is defined as the. This property is named after Pieter Zeeman a 20th-century physicist from the Netherlands who won the Nobel Prize in Physics and Hendrik Lorentz in 1902 to discover the effect.
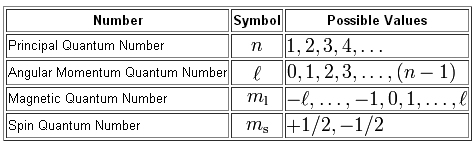
Quantum Numbers Introduction To Chemistry
Understanding the Zeeman effect has led to advancements in electron paramagnetic resonance studies and magnetic field measurements in space such as those of the Sun and other stars.

. GDL bears promise for molecular modelling applications that rely on. The electrons of the valence shell have less attraction to the nucleus and as a result can lose electrons more readily. The principal quantum number increases and average electron density moves farther from nucleus.
That is the electron affinity is equal to the energy difference between the enthalpy of formation of a neutral species and the enthalpy of formation of the negative ion of the same structure. Bells Theorem is the collective name for a family of results all of which involve the derivation from a condition on probability distributions inspired by considerations of local causality together with auxiliary assumptions usually thought of as mild side-assumptions of probabilistic predictions about the results of spatially separated experiments that conflict for. The nephelauxetic ratios β of Cr 3 complexes were found to be 0762 and that of Co 2 complexes was 0889 which indicates the covalency between the metal ions and their ligands.
Geometric deep learning GDL is based on neural network architectures that incorporate and process symmetry information. This causes an increase in metallic character. The electron affinity EA of a molecule is for negative ions or anions the quantity that is analogous to the ionization energy for positive ions.

Quantum Numbers The Easy Way Youtube

Principal Quantum Number An Overview Sciencedirect Topics

Quantum Numbers Principal Azimuthal Magnetic Videos And Examples
No comments for "The Principal Quantum Number Indicates What Property of an Electron"
Post a Comment